| 2-1. | Collagen & Gelatin as medical material |
We are supplying collagen and gelatin as medical materials, and are constantly striving to improve quality and safety.
| 2-2. | beMatrix gelatin |
Recently, highly purified gelatins have been required as materials for regenerative therapy related products. In response to customer demands, we have developed “bematrix gelatin” to meet the high safety and quality expectations in this field.
*beMatrix has been registered in the FDA device Master File.
- The breed is fixed
- Age is always the same
- Skin is harvested from the same part
- ISO9001
- IPEC GMP
- Cleanroom
- Endotoxin level is lower than 10 EU/g
- Virus inactivation is included in the production process.
|
| 2-3. | Collagen for medical application |
Since 20 years, we have been supplying highly purified types of collagen as a material for medical devices including wound dressing materials and bone void fillers. Furthermore in 2015, we established a larger production system and built a new and better clean room facility to meet the quality standards. The endotoxin level and viruses in our collagen products are reduced during the manufacture process. Please feel free to contact us about large supplies of collagen.
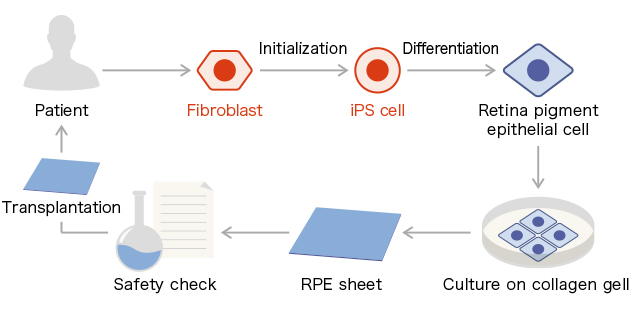
beMatrix collagen was developed in response to the demands in the field of regenerative medicine. It is a series of collagen solutions with reduced endotoxin and virus levels. beMatrixTM collagen AT was used as the culture material for retinal pigment epithelial cell sheets in the 1st clinical study on iPS cell-derived tissue transplantation conducted by Dr. Masayo Takahashi.